最高のコレクション balloon vinegar and baking soda 603205-Balloon vinegar and baking soda experiment hypothesis
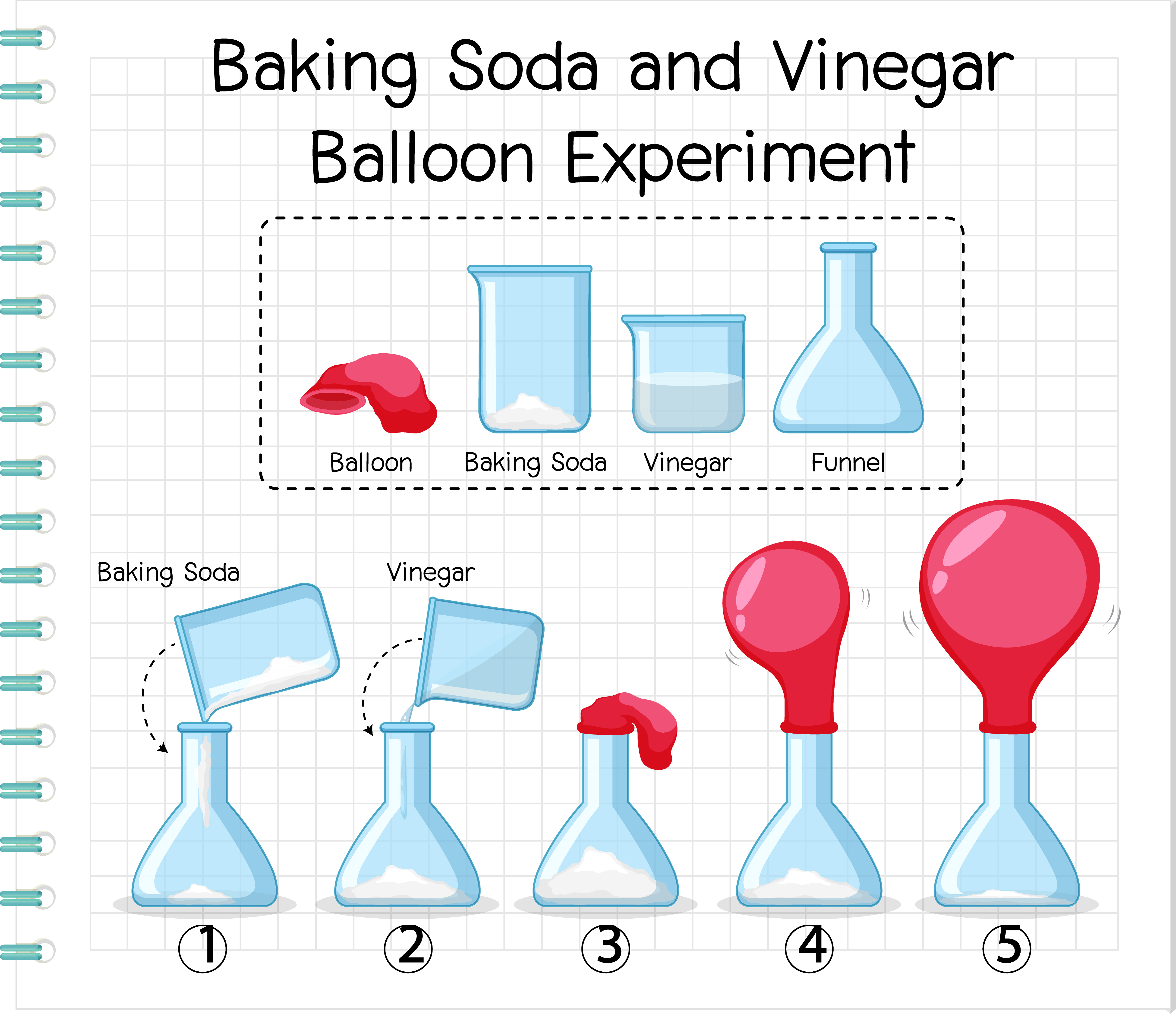
Are you ready to learn about chemical reactions? Step 1 Pour your vinegar into the bottle Set aside Step 2 Use your funnel and slowly measure in your two cups of baking soda Step 3 Take the bottle ring out of your bottle opening and add it over the balloon opening Step 4 Then snugly close the bottle opening with the balloon and add the ring back to secure in placeFunnel Instructions Use the funnel to add the 1/3 cup of baking soda into the balloon Twist the neck of the balloon a few times to keep the baking soda from spilling out and set the balloon aside Rinse the funnel and then use it to add the 1 cup of vinegar to the bottle

Use Vinegar And Baking Soda To Blow Up A Balloon Discovery Express
Balloon vinegar and baking soda experiment hypothesis
Balloon vinegar and baking soda experiment hypothesis-Pour two teaspoons of baking soda into your balloon, and pour half a cup of acetic acid into the bottle An intense reaction will begin, releasing CO2 and making the balloon inflate If the reaction is too weak to inflate the balloon, add more vinegar and baking soda , but don't shake the solutionSet her up for an afternoon of sciencefueled magic with a deceptively simple activity starring baking soda and vinegar Who knew science could be so easy, affordable, and fun?




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity
How to inflate a balloon with baking soda and vinegar Will the balloon float?★ DON'T FORGET TO SUBSCRIBE!Baking soda is bicarbonate (NaHCO3) and vinegar is acetic acid (HCH3COO) One of the products this reaction creates is carbon dioxide, which makes the bubbles When the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate Carbon dioxide is an important gas inGently and firmly holding the balloon on the bottle, turn it Baking soda falls into the vinegar, and begins a rapid chemical reaction resembling the volcanic eruption This produces carbon dioxide, creating pressure that inflates the balloon See how the liquid is foaming?
In this experiment, we're going to learn how to blow up a balloon using baking soda and vinegar!👉 MORE httHttp//bitly/1ZUlufx#socraticakids #socraticakidsscienceVinegar Baking Soda Balloons = FIZZY FUN!Kids Science Experiments S
Once the vinegar and baking soda mix, the balloon will start to automatically inflate For those inquisitive minds who want to know how this happens, the vinegar is an acid and baking soda is a base When you mix an acid with a base, it causes a chemical reaction In this reaction, carbon dioxide, which is a gas, is releasedThe baking soda and the vinegar create an ACIDBASE reaction and the two chemicals work together to create a gas, (carbon dioxide) Gasses need a lot of room to spread out and the carbon dioxide starts to fill the bottle, and then moves into the balloon to inflate it Vinegar and Baking Soda Balloon Does your curious chemist need some new inspiration?




Blowing Up Balloons With Baking Soda And Vinegar Pink Stripey Socks




How To Blow Up A Balloon With Baking Soda And Vinegar 9 Steps
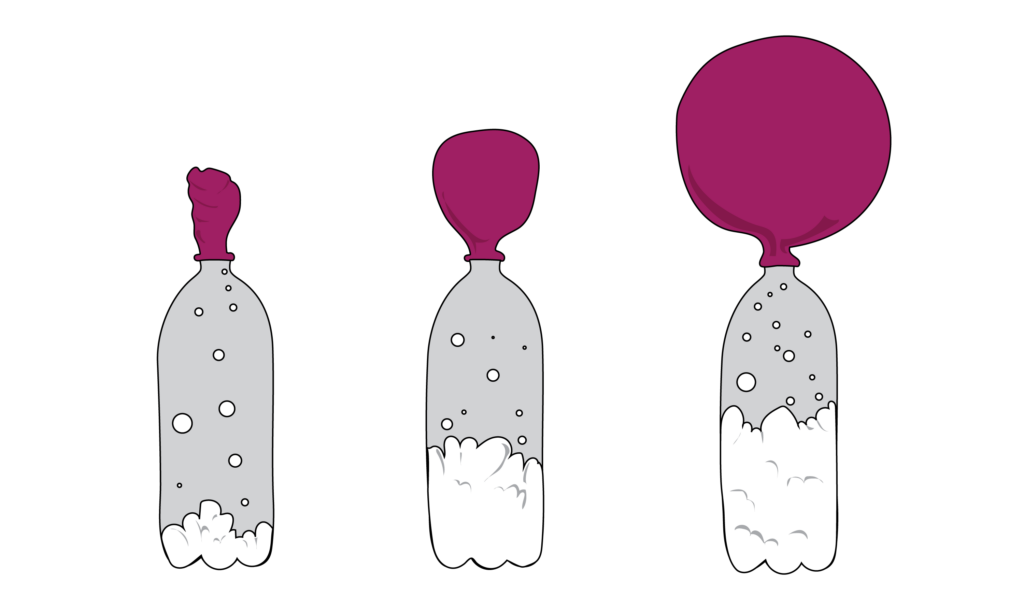
When baking soda and vinegar are mixed together, it creates a gas called carbon dioxide The gas begins to expand in the bottle and starts to inflate the balloon The more gas that is created, the larger the balloon will inflate Balloons Funnels Measuring spoon Start by putting the funnel into the balloon This will make it much easier to get the baking soda inside Add baking soda We added about 4 teaspoons to each balloon To get your balloons a bit bigger than ours, you could add 1 or 2 teaspoons more Next, use a funnel to pour some vinegar into your bottlesWhen vinegar and baking soda mix, they create the gas carbon dioxide and water The carbon dioxide has no where to go, but into the balloon blowing it up But you should have found that the balloon at room temperature may have been slightly faster in blowing up your balloon




How To Blow Up A Balloon With Baking Soda And Vinegar 9 Steps




Chemical Reaction Experiment When Baking Soda Stock Photo Edit Now
I took care of the prep work for the most part I put a shot or two of vinegar in each plastic bottle We had a set up for each of the kids I used a funnel to add a tablespoon of baking soda in each balloon Then tightly secured the balloon onto the top of the bottle without letting the baking soda dropBalloon 1 cup of vinegar; The science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients mix together the balloon baking soda experiment gets it's lift!



The Science Of Baking Soda Jerry James Stone




Balloon Baking Soda Vinegar Science Experiment For Kids
First use the funnel to pour about a cup of white vinegar into the water bottle Next place the funnel in the open end of the balloon and slowly pour about 2 teaspoons of baking soda into the balloon Place the open end of the balloon over the opening of the water bottle After the balloon is attached to the bottle, raise up the balloon until The baking soda and vinegar reaction can be used to produce sodium acetate, by boiling off or evaporating all the liquid water How the Reaction Works The reaction between baking soda and vinegar actually occurs in two steps, but the overall process can be summarized by the following word equationWhen baking soda and vinegar are mixed together, it creates a gas called carbon dioxide The gas begins to expand in the bottle and starts to inflate the balloon The more gas that is created, the larger the balloon will inflate How does baking soda and vinegar make a big reaction?




Balloon Baking Soda Vinegar Science Experiment For Kids




Mom To 2 Posh Lil Divas Blow It Up Exploring Gas With Balloons Baking Soda Vinegar
what causes a balloon to inflate with baking soda and vinegar? How to inflate a balloon with vinegar and baking soda Tip a small amount of baking soda into one balloon and larger amount of baking soda into the other balloon This is quite tricky – adults may have to help hold the balloon open so kids can get the baking soda into the balloon Give the balloons a shake so all the baking soda falls to the The science and explanation to this baking soda balloon experiment The baking soda, and vinegar are mixing together to form a gas called carbon dioxide The gas expands It needs room to expand and grow If the water bottle outlet is sealed, the gas has no choice but to travel up and out into the balloon, filling the spaces up and inflating it!




Blow Up A Balloon With Baking Soda And Vinegar Frugal Fun For Boys And Girls




Vinegar And Baking Soda Balloon Experiment Happy Brown House
Directions Pour ARM & HAMMER Baking Soda into your balloon, filling the balloon halfway Use the funnel to pour vinegar into the water bottle, filling about ⅓ of the bottle Cover the top of the bottle with the bottom of the balloon When ready, lift the balloon and let the ARM & HAMMER Baking Soda fall into the vinegar Step 1 Add 2 or 3 teaspoons of baking soda to the unfilled balloon Step 2 Thoroughly rinse and dry the funnel Step 3 Pour 2 or 3 teaspoons of vinegar to the water bottle using the funnel Step 4 Attach the balloon to the top of the water bottle, being careful to not let any of the baking soda slip in until you are readyBalloon Chemical Change Experiment, Chemical Change Lab In this chemical change lab, students will observe how baking soda and vinegar react in a plastic bottle to blow up a balloon and produce a chemical change Teachers will need to provide vinegar, baking soda, a balloon, and an empty water bot




Blow Up Balloon Activity Science Museum Group Learning




Vinegar And Baking Soda Balloon Activity Education Com
Vinegar inside the bottle is an acid, and baking soda added from the balloon is a base When they mix together, sodium acetate and carbonic acid are formed This carbonic acid separates into carbon dioxide and water So there is a buildup of carbon dioxide gas inside the bottle The baking soda and vinegar reaction is actually two separate reactions The first reaction is the acid base reaction When vinegar and baking soda are first mixed together, hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking sodaEither a small funnel or a piece of paper that you can roll into a cone Carefully pour about a 1/4 cup of vinegar into your plastic bottle and use a small funnel to put a spoonful or two of baking soda into your balloon Don't have a funnel?




Blowing Up Balloons With Baking Soda And Vinegar Pink Stripey Socks




Baking Soda And Vinegar Balloon Experiment
Pour two teaspoons of baking soda into your balloon, and pour half a cup of acetic acid into the bottle An intense reaction will begin, releasing CO2 and making the balloon inflate If the reaction is too weak to inflate the balloon, add more vinegar and baking soda , but don't shake the solutionThis is how my little scientist inflates balloons!Baking soda in balloons and vinegar with food colouring in bottles=====1/3 cup of baking soda;




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do



Koch Newsroom Baking Soda Balloons
The vinegar and the baking soda mix together to make an acidbase reaction The reaction creates carbon dioxide gas that bubbles up from the mixture The gas expands up and out of the bottle and inflates the balloon What is the chemical reaction in baking soda and vinegar? Attach a balloon to the end of the funnel Using the funnel, pour two level teaspoons (10 mL) of baking soda into the balloon (see photo below) (Make sure the funnel doesn't clog, and all the baking soda passes through the neck of the balloon) Pour about 1/2 cup (1 mL) of vinegar into the bottle or flaskFor this experiment you need a few things 1 Baking Soda 2 Vinegar 3 oz pop bottle 4 Spoon 5 Funnel 6 Balloon 7 Clothes Pin
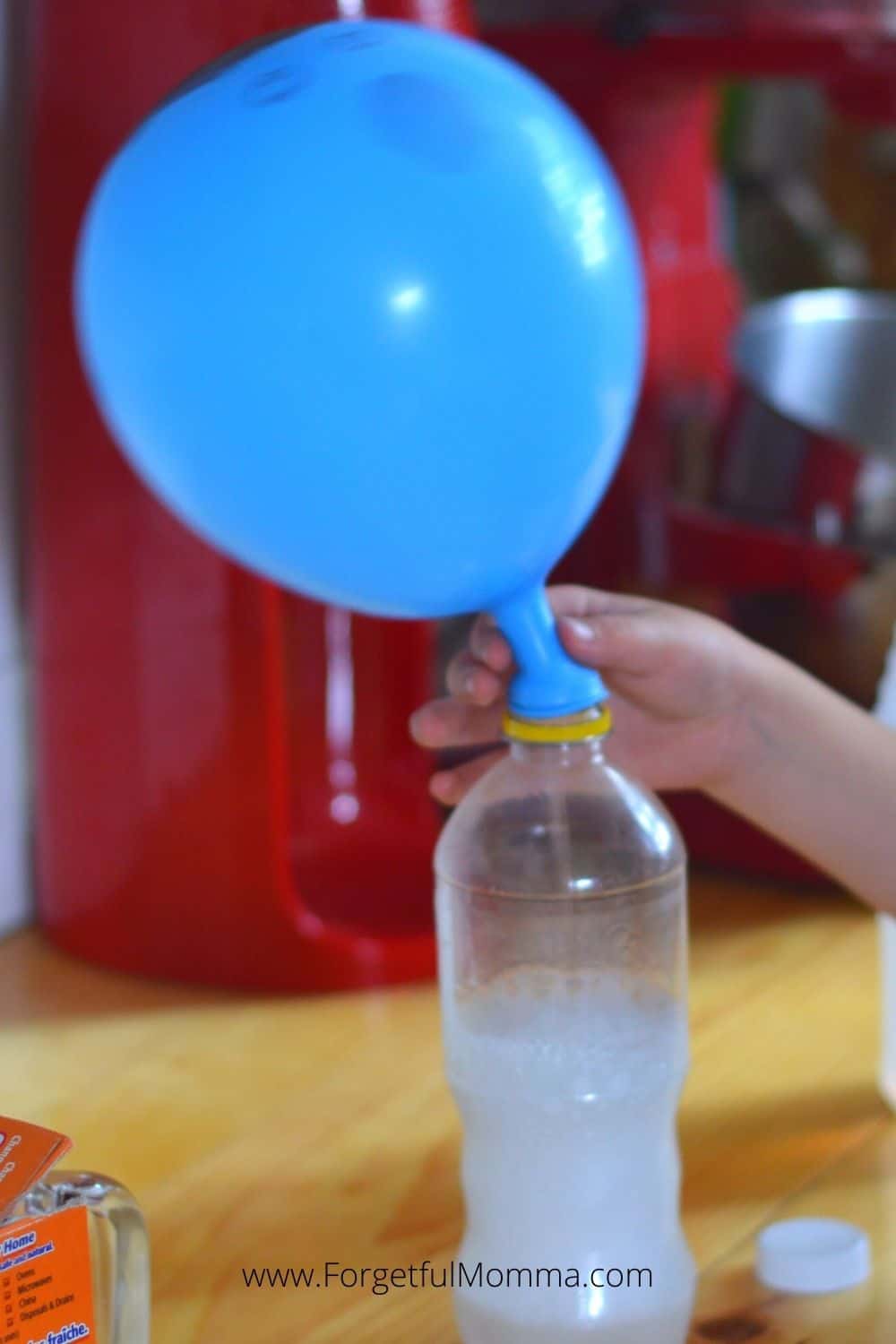



Self Inflating Balloon Experiment Forgetful Momma




Baymax S Low Battery Baking Soda And Vinegar Experiment
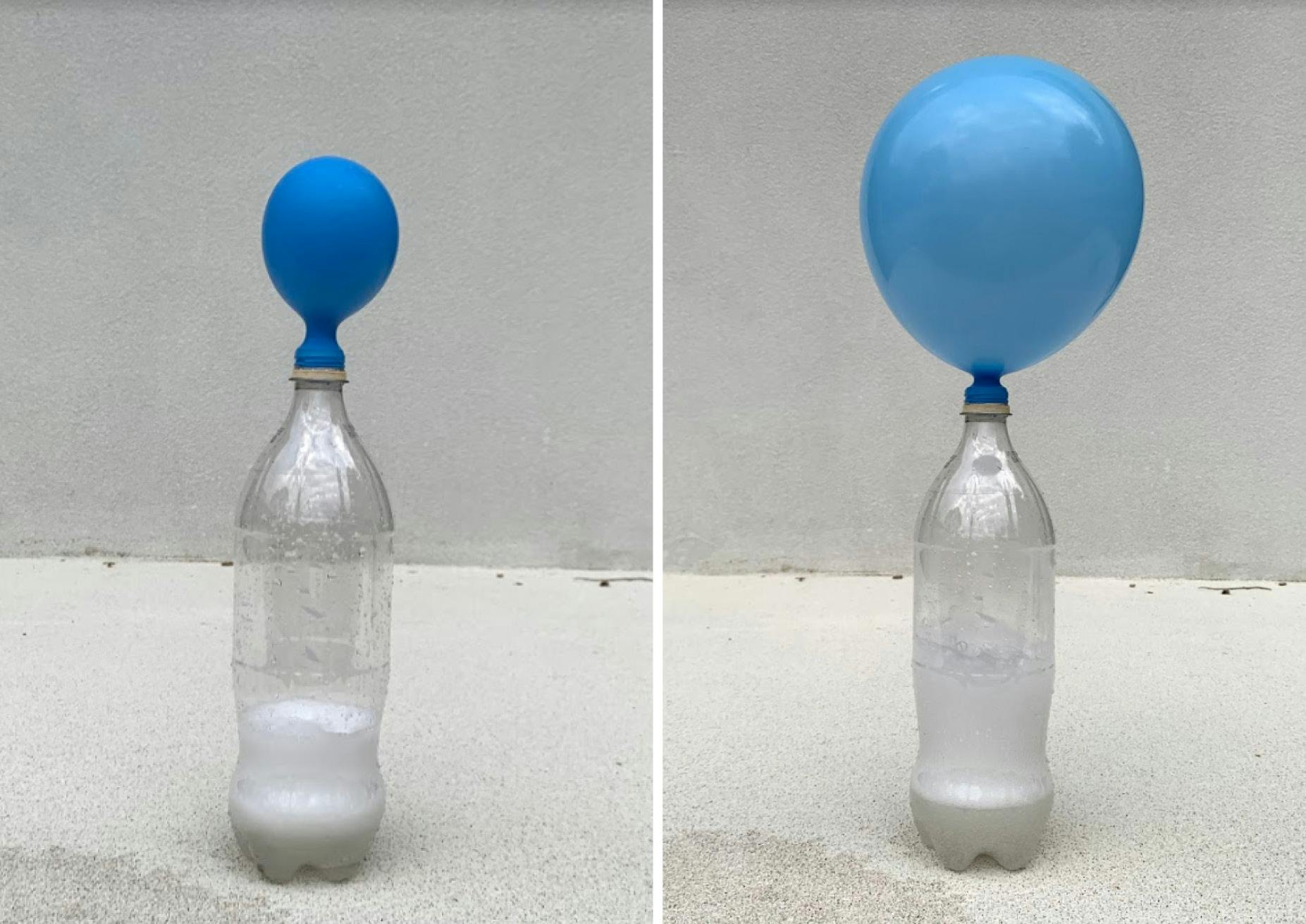
Fill the soda bottle with 1 cup of vinegar Cut a small corner from the clear bag and add ¼ tsp of baking soda into the bag fragment as shown below Carefully, drop the small bag into the soda bottle with the corner of the bag pointedHttp//bitly/SubtoMysteryLab ★Hey Everyone and we Pour the vinegar into the bottle Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar Observe the chemical reaction and effect on the balloon




Possibly Messy Science Activities To Do With Kids Tulsakids Magazine




How To Blow Up A Balloon With Vinegar And Baking Soda Or Yeast
Baking Soda Balloons by Bryce Hixson Great lab to teach conservations of mass during a chemical reaction Vinegar is placed in a balloon, which is fixed over a test tube with baking soda The assemblage is weighed before and after the chemicals are mixedTweet about this video! The baking soda/vinegar balloons is a fascinating demonstration of acid base chemistry Vinegar is water with about 3 percent of a chemical called Acetic acid Baking Soda is a compound called Sodium Bicarbonate, also known as Sodium Hydrogen Carbonate (NaHCO 3), and is a base So the reaction occurs




Blow Up A Balloon With Baking Soda And Vinegar Happy Brown House




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity
Vinegar, Baking Soda, and a Balloon!1 Using your funnel pour vinegar into your bottle 2 Using another (dry) funnel pour baking soda into your balloon Fill the balloon approx 1/2 way 3 Cover the top of the bottle with you balloon Make sure you don't let the baking soda spill into the bottle prematurely Watch as the mixture fizzes, bubbles & expands your balloonPour the baking soda into the balloon using a funnel Measure 45 ml of vinegar and pour it into a water bottle Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon (The balloon will be flopped to one side) Lift the balloon up and pour the baking soda into the bottle of vinegar Repeat for each type of vinegar




Fizzy Fun 5 Baking Soda Experiments Teaching Mama



Science With Children Baking Soda And Vinegar Experiments
Baking Soda VS Vinegar With Balloon #Baking Soda #Shorts #Vinegar #Balloon #Experiment #No limit Experiments When the vinegar and baking soda combine there is a reaction between an acid and a base Vinegar is the acid and baking soda is the base This reaction between the two causes a gas called carbon dioxide to bubble and foam This gas having nowhere else to go, expands the balloon making the selfinflating balloon happen Want More Baking Soda and When baking soda and vinegar are mixed together, it creates a gas called carbon dioxide The gas begins to expand in the bottle and starts to inflate the balloon The more gas that is created, the larger the balloon will inflate




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




Simple Science Experiment Chemical Reaction Brigitte Brulz
That lift is the gas produced from the two ingredients is carbon dioxide or CO2




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity




Build A Fizz Inflator Sciencebob Com



Blowing Up Balloons With Co2 A Unique Hands On Science Night




Blow Up A Balloon With Baking Soda And Vinegar Frugal Fun For Boys And Girls




Baking Soda And Vinegar Balloon Experiment




Blowing Up Giant Balloon Baking Soda And Vinegar Experiment For Kids Youtube



1



Baking Soda Vinegar Balloon Experiment Capturing Parenthood




Balloon Baking Soda Vinegar Kids Science




Blowing Up A Balloon With Baking Soda And Vinegar Cool




Baking Soda And Vinegar S Reaction Perkins Elearning




Baking Soda And Vinegar Balloons



1




Baking Soda And Vinegar Balloon Experiment Balloon Blowing Up 2 Happy Brown House




Experiments With Baking Soda And Vinegar Thriftyfun




Baking Soda And Vinegar Balloon Experiment Science Project Education Com




Inflate A Balloon With Baking Soda And Vinegar Pbs Kids For Parents




How To Make Balloon Inflated With Baking Soda And Vinegar Diy Crafts Handimania




How To Make A Heavy Balloon We All Know That Balloons When Inflated With Helium Float Because Helium Is Lighter Than Air But Have You Ever Thought Ppt Download




Create Carbon Dioxide Boundless Brilliance




Balloon Blow Up Science Experiment



Science Experiments For Kids Blow Up A Balloon With Vinegar And Baking Soda



Theimag Org Wp Content Uploads 03 Baking Soda And Vinegar Balloon 1 Pdf
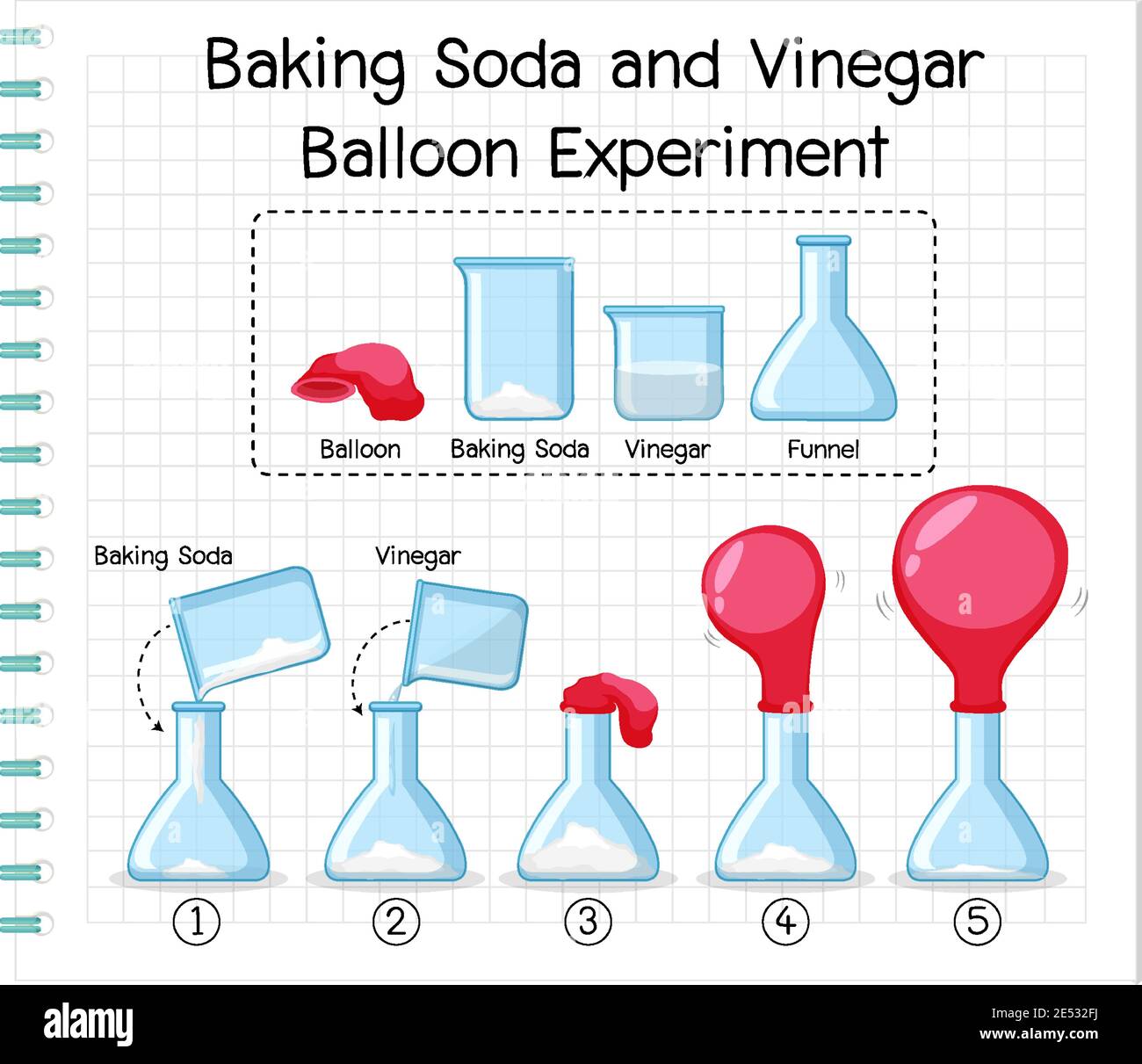



Science Experiment With Baking Soda And Vinegar Balloon Illustration Stock Vector Image Art Alamy
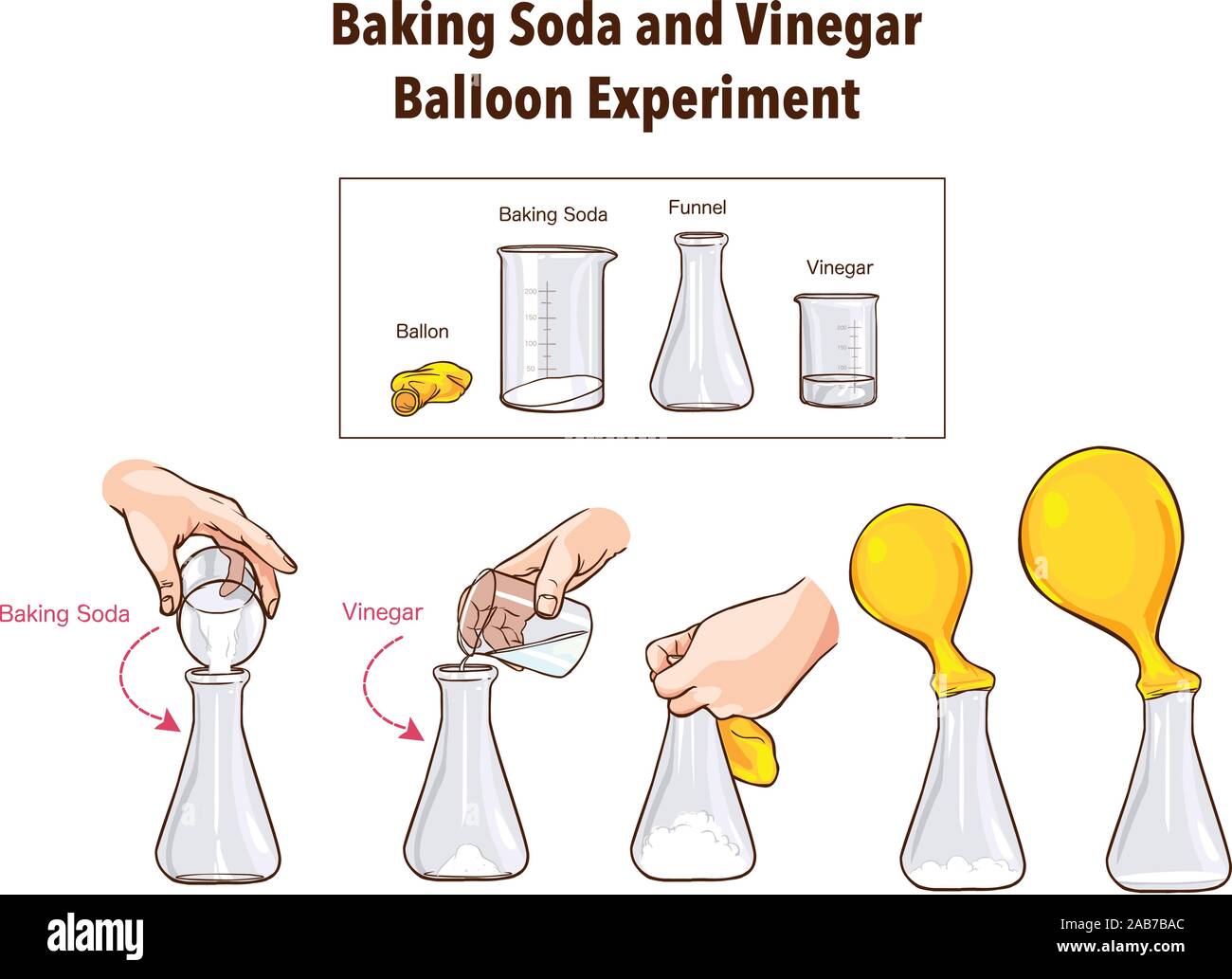



Baking Soda And Vinegar Balloon Experiment Science Stock Vector Image Art Alamy




How To Blow Up A Balloon With Baking Soda And Vinegar Jm Cremp S Adventure Blog




Mom To 2 Posh Lil Divas Blow It Up Exploring Gas With Balloons Baking Soda Vinegar




Baking Soda And Vinegar Balloons




How To Inflate A Balloon Using Baking Soda And Vinegar




Baking Soda Vinegar And Our Atmosphere Experiment Wltx Com




Day 15 Baking Soda And Vinegar Balloon Bombs Operation Summer Lovin




Wait Weight Don T Tell Me Chemistry Earth Science Science Activity Exploratorium Teacher Institute Project




Balloon Magic With Baking Soda And Vinegar Gift Of Curiosity




How To Inflate Balloon With Vinegar And Baking Soda Science4fun




Baking Soda And Vinegar Balloon Experiment Science Projects For Kids Educational Videos By Mocomi Youtube




Scientist Squad Diy Baking Soda And Vinegar Science Experiments For Kids Blowing Balloon Valcano Balloon Magic Facebook




Baking Soda And Vinegar Balloons




Blowing Up A Balloon With Baking Soda And Vinegar Cool




How To Blow Up A Balloon With Baking Soda And Vinegar At Home Science Experiments For Kids Jm Cremp S Adventure Blog




Baking Soda And Vinegar Balloon Experiment Balloon Blowing Up 1 Happy Brown House




Magic Balloon Inflation Baking Soda And Vinegar Experiment Midwestern Mama




Balloon Baking Soda Vinegar Science Experiment For Kids




Use Vinegar And Baking Soda To Blow Up A Balloon Discovery Express




Baking Soda And Vinegar Balloons




Balloon Baking Soda Vinegar Kids Science




Fun Experiment To Do With The Kids Inflating A Balloon With Bicarbonate Of Soda And White Vinegar




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




Blowing Up A Balloon With Baking Soda And Vinegar Thriftyfun




Baking Soda Vinegar Balloon Experiment Easy Fun Kid Friendly Things To Do




Baking Soda Vinegar Balloon Experiment Youtube




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Build A Fizz Inflator Sciencebob Com




Balloon Baking Soda Vinegar Experiment Gagmad



Science Experiments For Kids Blow Up A Balloon With Vinegar And Baking Soda




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities



1




Kid Science Hot Air Balloon Bottle Kix Cereal




Baking Soda And Vinegar Balloon Experiment For Kids Baking Soda Experiments Balloon Science Experiments Kid Experiments




Baking Soda And Vinegar Balloon Experiment Youtube



Easy Baking Soda Balloon Experiment




Baking Soda And Vinegar Balloon Experiment For Kids Science Experiments Kids Kid Experiments Balloon Experiment




Baking Soda And Vinegar Balloon Experiment Science Royalty Free Cliparts Vectors And Stock Illustration Image




Blow Up Balloon This Time With Baking Soda And Vinegar Science And Samosa




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful



Science Experiment With Baking Soda And Vinegar Balloon Vector Art At Vecteezy



3




This Self Inflating Balloon Experiment Is Awesome By Kidadl



At Home Lesson Breathless Balloons With Edie S Experiments Penguin Books Australia




Bullshit Make Balloons Float With Vinegar And Baking Soda Isitbullshit




Science Museum From Bubbles To Balloons How Do You Blow Up A Balloon Without Using Your Breath Or A Pump With Science Of Course Using Water Vinegar Baking Soda




Mama S Little Muse Science Activity Blowing Up A Balloon Using Baking Soda And Vinegar




Baking Soda And Vinegar Balloons




How To Inflate A Balloon With Baking Soda Vinegar Stemglobe




How To Fill A Balloon With Baking Soda Vinegar 5 Steps With Pictures Instructables




Giant Balloon Baking Soda And Vinegar Experiment Hello Wonderful




Baking Soda Vinegar Balloon Experiment The Go To List



Blow Up Balloons With Baking Soda And Vinegar Just A Pinch Recipes




Baking Soda Vinegar And Our Atmosphere Experiment Wltx Com
コメント
コメントを投稿